Category: Cancer
Seerave invests in Florey Biosciences and takes aim at mitigating impacts of antibiotic use

Key Points
- Seerave Ventures has made a pre-seed investment in Florey Biosciences, a spinout of the Wyss Institute at Harvard University
- Florey is developing an engineered yeast strain designed for the management of antibiotic damage to the gut microbiome
- As a founding partner, Seerave will contribute strategic and operational support alongside its capital investment
October 1, 2024 – Today, we are announcing that Seerave Ventures, the investment arm of the Foundation, has made a new investment in Florey Biosciences, a synthetic biology company with a novel technology platform that can deliver any protein of interest to the gut. Florey’s lead program is a medical food intended for the dietary management of antibiotic damage to the gut microbiome.
Florey’s technology is a promising innovation for addressing the impacts of antibiotic use. It speaks to the strategic commitment that we recently announced to mitigate the impacts of microbiome modifiers on cancer outcomes and deliver meaningful benefits to patients within five years.
Immunotherapy is one of the most promising new frontiers of cancer treatment, but overall survival is about half as long for patients who received antibiotics before immunotherapy. Often, taking antibiotics is an unavoidable and life-saving measure. It can, however, compromise the microbes that live in our guts and that play a profound role in how our immune systems function.
“We see our support of Florey as a key part of our efforts to meaningfully improve patients’ lives within the next five years,” said Manuel Fankhauser, CEO of the Seerave Foundation, in a press release issued by Florey on Oct 1.
As part of Seerave’s involvement, Mark Smith, Venture Lead at Seerave, has joined the board of Florey Biosciences.
For more information:
Continue Reading
Related Articles
Seerave Announces Strategic Focus on Antibiotics and Cancer Immunotherapy
Over the past twelve months, the Seerave Foundation has convened large parts of its network to identify how to best position its science and venture philanthropy platform to deliver meaningful improvements to cancer care within the next five years. Drawing from these conversations, which have been partially summarized in a recent Cancer Cell perspective, we would like to announce a new strategic focus for the Foundation: mitigating the impacts of antibiotics and other microbiome modifiers on cancer patients’ outcomes.
An estimated 30% of patients take antibiotics prior to immunotherapies like immune checkpoint inhibitors, and analyses of more than 40,000 patients have found a strong association between antibiotic use, immune system function, and poorer responses to these promising new treatments. Immunotherapies still only work in a fraction of cancer patients, and it has become a major effort of oncology researchers to uncover why.
Members of Seerave’s research network put forth some of the first studies and meta analyses looking closely at the relationship between antibiotic use, immunotherapy, and poorer patient outcomes, and the follow-up investigations on mechanism and causality.
Building on this work, Seerave’s strategic focus is designed to help the Foundation measurably improve cancer patients’ lives within five years. Seerave aims to clarify how antibiotics and other microbiome modifiers impact patient outcomes, inform practical advice to clinicians and patients, and propel the development of new tools and technologies.
In pursuit of these goals, we are privileged to welcome our first Scientific Advisors, Martin Blaser, MD and Per Falk, MD, PhD to advance the Foundation’s research agenda and bring scientific excellence to its programs.
- Dr. Martin Blaser holds the Henry Rutgers Chair of the Human Microbiome at Rutgers University, and serves as Director of the Center for Advanced Biotechnology and Medicine. A physician and microbiologist, Dr. Blaser has driven seminal research on the relationship of the human microbiome with health and diseases, including asthma, obesity, diabetes, and cancer. Dr. Blaser has served as President of the Infectious Diseases Society of America, Chair of the Board of Scientific Counselors of the National Cancer Institute, Chair of the Advisory Board for Clinical Research of the NIH, and Chair of the Presidential Advisory Council for Combatting Antibiotic Resistant Bacteria (PACCARB). He has authored over 600 original scientific articles, holds 24 U.S. patents, and is the author of Missing Microbes.
- Dr. Per Falk has been investigating and advancing microbiome science for over 30 years, most recently as President and Chief Scientific Officer of Ferring Pharmaceuticals, where he oversaw the development of one of the first microbiome therapies to receive FDA approval. Prior to joining Ferring, Dr. Falk held executive and senior leadership positions at Novo Nordisk and AstraZeneca in research, medical and clinical development roles. He has held the role of Associate Professor at the Karolinska Institute, Sweden and the Washington University School of Medicine, USA.
Seerave is also delighted to welcome two new team members, Mark Smith, PhD and Carolyn Edelstein, to lead some of its new activities. Our approach will be organized under the following strategic pillars:
- Data Integration: Build a data consortium that collaboratively integrates and interrogates clinical data on microbiome modifiers in cancer immunotherapy from leading hospitals and research institutions. Led by Laura Mählmann
- Policy and Practice: Empower oncologists and other key stakeholders with practical, evidence-based advice that reflect the latest understanding of the role of microbiome modifiers in cancer care. Led by Carolyn Edelstein
- Novel Tools: Foster the development of new tools and services for oncologists through Seerave Ventures, our hands-on impact investment vehicle that supports a select number of mission-aligned companies with the potential to transform patient care. Seerave Ventures will be led by Mark Smith
- Community and Collaboration: Cultivate community across disciplinary silos and geography by creating and empowering a self-sustained, mission-aligned network of relevant stakeholders, including clinical researchers, clinical practitioners, public health experts, entrepreneurs, and patient advocates. Led by Laura Mählmann
A legacy of bringing new insights to cancer care
Almost a decade ago, after seeing how an emphasis on microbiome health brought new hope during his wife’s cancer journey, David Rees established the Seerave Foundation with a vision of bringing precision medicine and groundbreaking scientific insights on the microbiome and immune system function to cancer care. At the time, an appreciation of the microbiome’s relevance to cancer was far from mainstream. Today, it is seen as one of the most promising new arenas in the search for therapeutic breakthroughs. Seerave’s research network has driven many of the insights that have mobilized the field, including:
- The Mediterranean diet is associated with improved outcomes in melanoma patients following immune-checkpoint blockade [1]
- The microbiome is different in responders vs. non-responders to immune-checkpoint blockade [2] [3], there are plausible mechanisms by which microbiota may influence therapeutic response, and biomarkers of cancer-associated microbiome dysbiosis [4]
- Fecal microbiota transplantation may improve immune-checkpoint-blockade outcomes [5]
Beyond its role in catalyzing research, Seerave has supported the translation of these and other key discoveries into a wide array of products, from new public health tools and biotechnology companies to public interest projects like microbiome conservation initiatives and critically acclaimed documentaries.
We look forward to sharing updates of our progress in the coming months. We would also keenly welcome feedback and opportunities to collaborate, and we would love to hear if our mission and work resonates with you and is as close to your heart as it is to ours.
Continue Reading
Related Articles
More effective cancer immunotherapy thanks to the gut microbiome
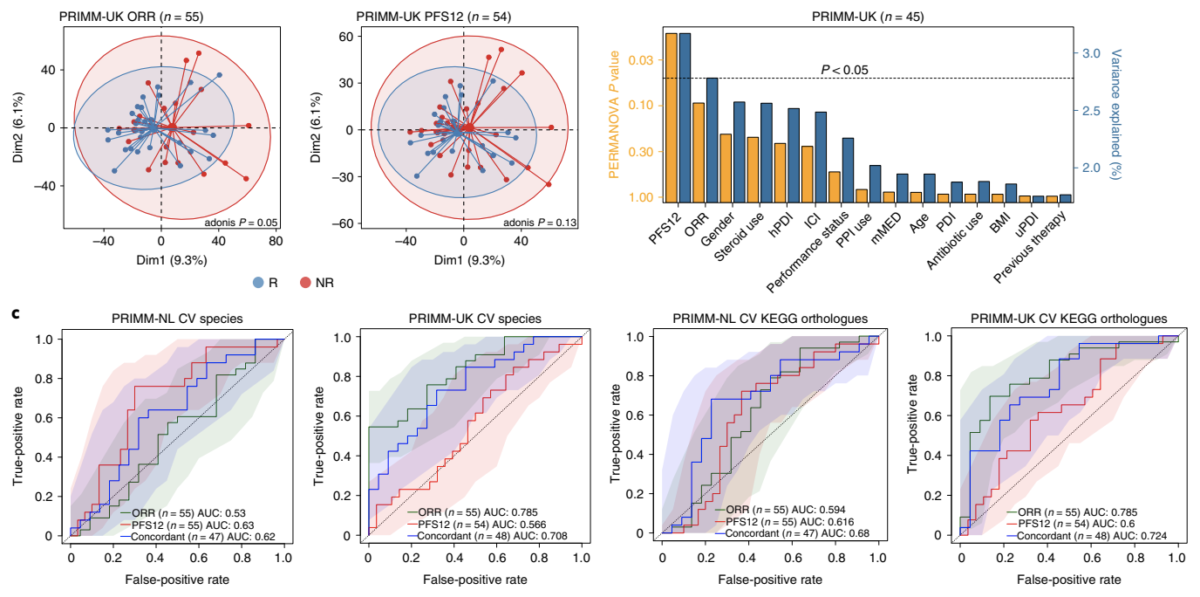
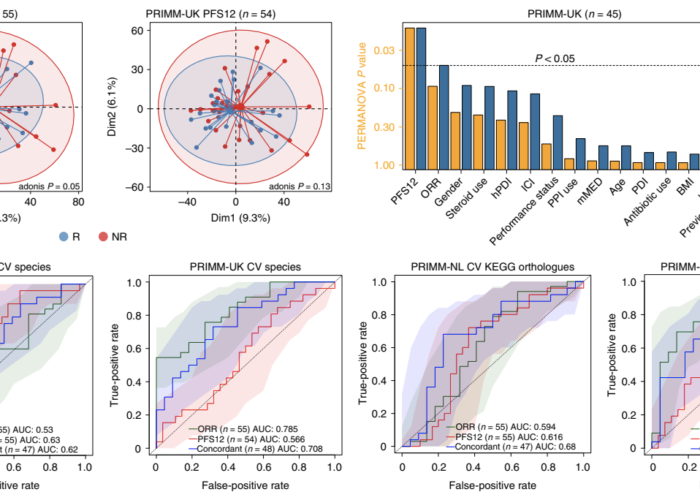
For more than four years, Seerave Foundation has been supporting the outstanding research teams at King’s College London (UK), UMCG (NL) and University of Trento (I) to find out whether there is a link between the presence and function of the gut microbiome and the outcome of cancer immunotherapy. We are thrilled to see this milestone work being published in Nature Medicine!
Congrats to all the authors and especially Karla Lee, Andrew Thomas, Laura Bolte, Johannes Björk, Laurence Zitvogel, Veronique Bataille, Geke Hospers, Tim Spector, Rinse Weersma and Nicola Segata from the Seerave network!
In summary, the team found that the gut microbiome has a relevant, but cohort-dependent, association with the response to ICIs. A panel of species, including Bifidobacterium pseudocatenulatum, Roseburia spp. and Akkermansia muciniphila, associated with responders was identified, but no single species could be regarded as a fully consistent biomarker across studies. Overall, the role of the human gut microbiome in ICI response appears more complex than previously thought, extending beyond differing microbial species simply present or absent in responders and non-responders.
We hope that this open access publication will drive more research into finding the mechanistic answers needed to drive personalised microbiome-targeted interventions forward. Dedicated papers regarding the association of different diet patterns with response in the same cohorts will be published separately in the coming months (as well as papers including proteomic, glycomic and metabolomic analysis).
Stay tuned!
Source: https://www.nature.com/articles/s41591-022-01695-5.pdf
Continue Reading
Related Articles
The gut microbiome: what the oncologist ought to know
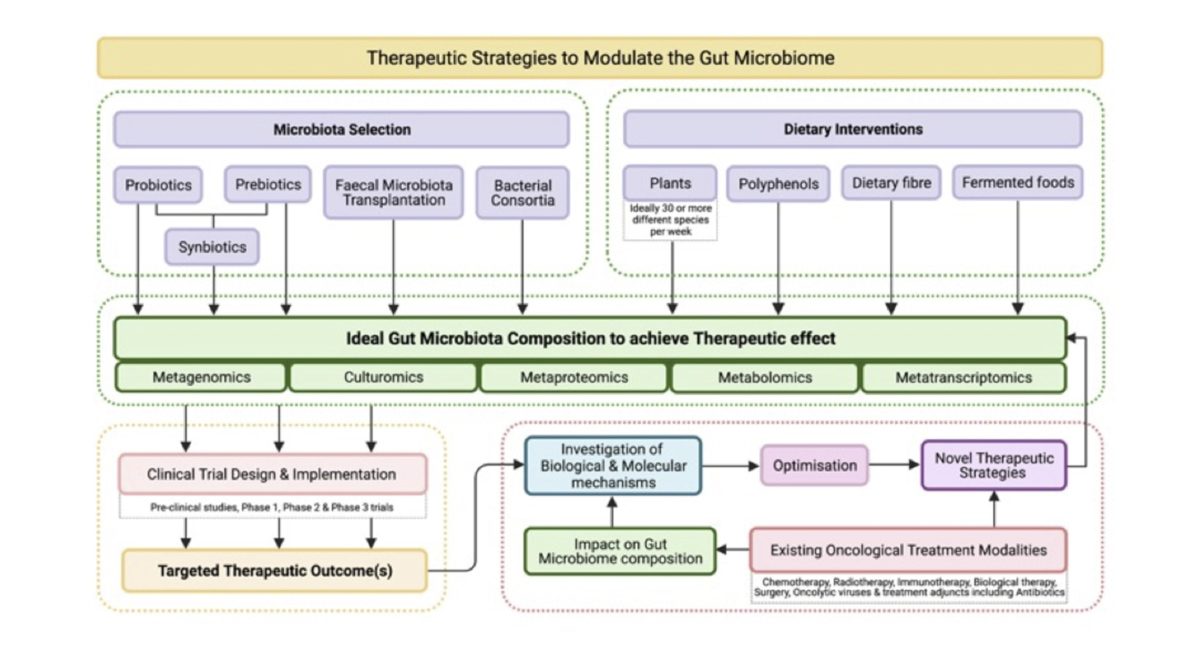
The gut microbiome (GM) has been implicated in a vast number of human pathologies and has become a focus of oncology research over the past 5 years. The normal gut microbiota imparts specific function in host nutrient metabolism, xenobiotic and drug metabolism, maintenance of structural integrity of the gut mucosal barrier, immunomodulation and protection against pathogens.
Strong evidence is emerging to support the effects of the GM on the development of some malignancies but also on responses to cancer therapies, most notably, immune checkpoint inhibition.
Tools for manipulating the GM including dietary modification, probiotics and faecal microbiota transfer (FMT) are in development. Current understandings of the many complex interrelationships between the GM, cancer, the immune system, nutrition and medication are ultimately based on a combination of short‐term clinical trials and observational studies, paired with an ever-evolving understanding of cancer biology.
The next generation of personalised cancer therapies focusses on molecular and phenotypic heterogeneity, tumour evolution and immune status; it is distinctly possible that the GM will become an increasingly central focus amongst them.
The aim of this review is to provide clinicians with an overview of microbiome science and our current understanding of the role the GM plays in cancer.
Continue Reading
Related Articles
Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade
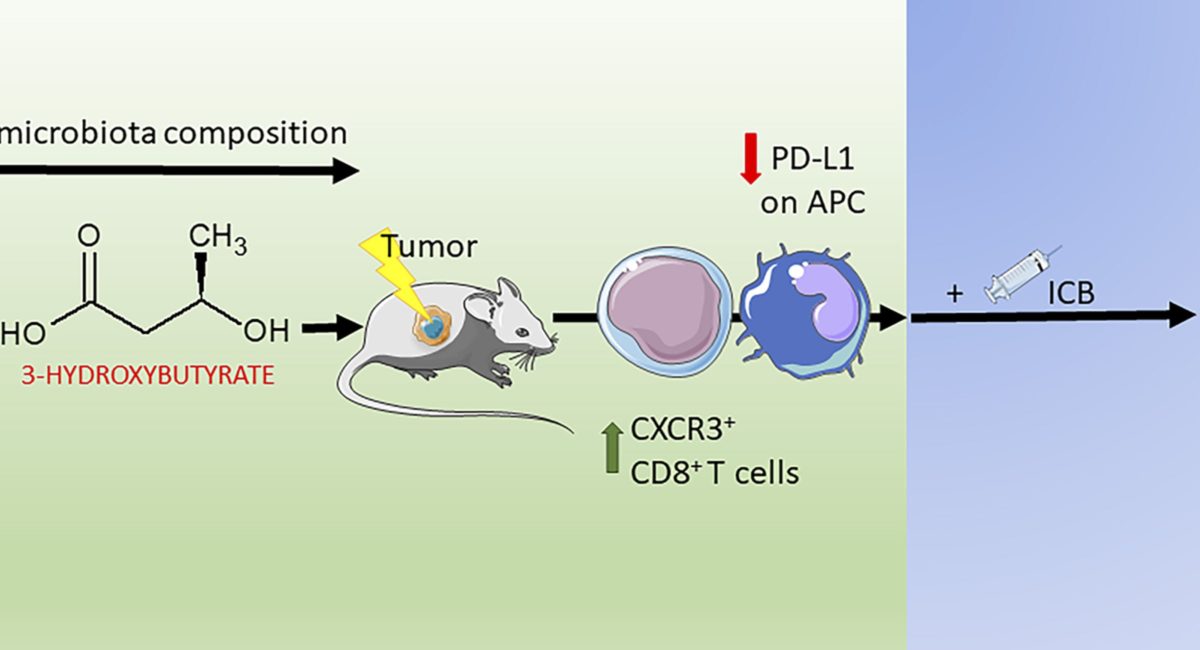
Limited experimental evidence bridges nutrition and cancer immunosurveillance. Here, the team at IGR Paris shows that ketogenic diet (KD) or its principal ketone body, 3-hydroxybutyrate (3HB), most specifically in an intermittent scheduling, induced T cell-dependent tumour growth retardation of aggressive tumour models.
In conditions in which anti-PD-1, alone or in combination with anti-CTLA-4, failed to reduce tumour growth in mice receiving a standard diet, KD or oral supplementation of 3HB reestablished therapeutic responses. Supplementation of KD with sucrose (which breaks ketogenesis, abolishing 3HB production) or with a pharmacological antagonist of the 3HB receptor GPR109A abolished the anti-tumour effects. Mechanistically, 3HB prevented the ICB-linked up-regulation of PD-L1 on myeloid cells while favouring the expansion of CXCR3+ T cells. KD induced compositional changes of the gut microbiota with distinct species such as Eisenbergiella massiliensis commonly emerging in mice and humans subjected to carbohydrate low diet interventions and highly correlating with serum concentrations of 3HB. Altogether, these results demonstrate that KD induces a 3HB-mediated antineoplastic effect that relies on T-cell mediated cancer immunosurveillance.
Continue Reading
Related Articles
Gut microbiome linked to efficacy of PD-1-inhibitor therapy for solid cancers
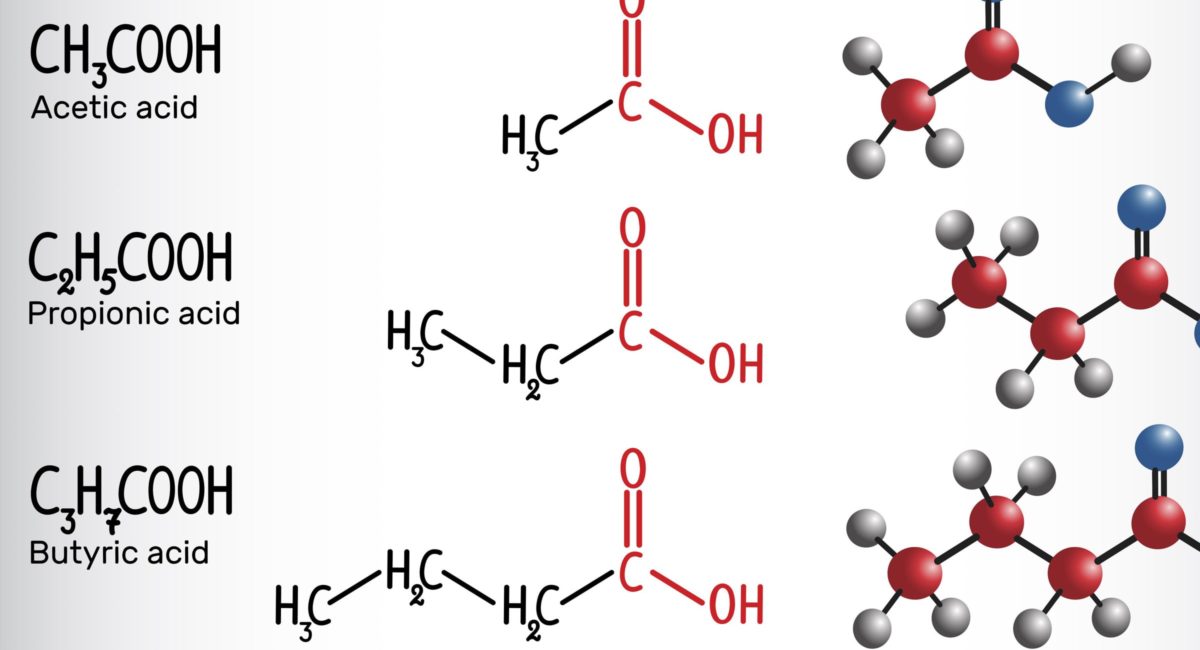
In patients with solid cancers, the concentration of fecal short-chain fatty acids (SCFA) may be a biomarker of the efficacy of the programmed cell death 1 (PD-1) checkpoint inhibitors nivolumab and pembrolizumab, according to researchers in Japan.
Immune-checkpoint inhibitors have been remarkably effective across multiple cancer types, note Dr. Motoo Nomura of Kyoto University and colleagues in JAMA Network Open. However, for solid cancers the response rate to PD-1 inhibitors has been relatively low, they add.
Thus, a biomarker of efficacy “is critically needed for clinical decision-making,” they say, and the gut microbiome profile could be one such factor.
To investigate, the researchers prospectively studied 52 cancer patients with a median age of 67 years who were scheduled to be treated with nivolumab or pembrolizumab. Concentrations of SCFAs in fecal and plasma samples were determined before PD-1 inhibitor administration.
The overall response rate was 28.8% and the median follow-up of survivors was for two years. There were no significant differences between responders and nonresponders in patient characteristics,
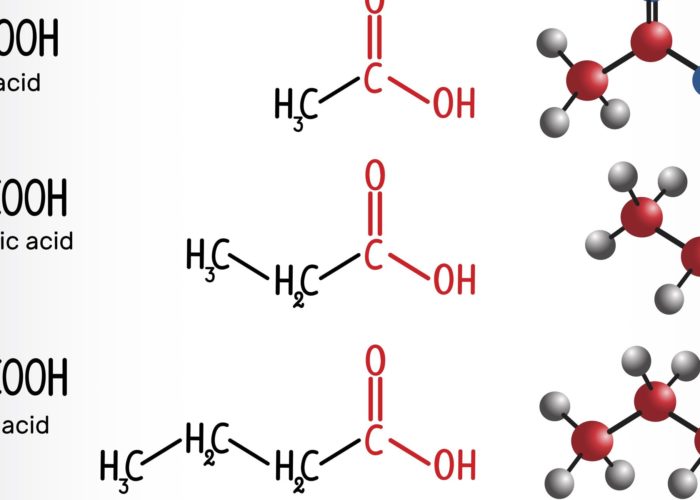
However, concentrations of fecal and plasma SCFAs were higher in the responder than nonresponder group, and high concentrations of some SCFAs were significantly associated with longer progression-free survival. These included fecal acetic acid (hazard ratio, 0.29), propionic acid (HR, 0.08) and butyric acid (HR, 0.31). This was also the case for plasma isovaleric acid (HR, 0.38).
The results, the researchers say, “showed that high frequencies of intake of several sources of dietary fiber, such as green vegetables, cabbage, and mushrooms, were associated with high concentrations of fecal SCFAs.”
There was no significant association between green vegetable or cabbage intake and progression-free survival. However, Dr. Nomura told Reuters Health by email, “A high frequency of mushroom intake during the one year preceding the onset of their current cancer was significantly associated with longer progression-free survival (HR, 0.40) in patients with solid cancer tumors treated with programmed cell death-1 inhibitors.”
However, he and his colleagues stress that the dietary information used in their study was collected before it started.
The researchers call for further studies but suggest that SCFAs may be the link between the gut microbiota and PD-1-inhibitor efficacy.
“Because fecal examinations are completely noninvasive, they may be applicable for routine monitoring of patients,” they say.
Continue Reading
Related Articles
Microbial DNA in patient blood may be tell-tale sign of cancer
When Gregory Poore was a freshman in college, his otherwise healthy grandmother was shocked to learn that she had late-stage pancreatic cancer. The condition was diagnosed in late December. She died in January.
“She had virtually no warning signs or symptoms,” Poore said. “No one could say why her cancer wasn’t detected earlier or why it was resistant to the treatment they tried.” As Poore came to learn through his college studies, cancer has traditionally been considered a disease of the human genome — mutations in our genes allow cells to avoid death, proliferate and form tumors.
But when Poore saw a 2017 study in Science that showed how microbes invaded a majority of pancreatic cancers and were able to break down the main chemotherapy drug given to these patients, he was intrigued by the idea that bacteria and viruses might play a bigger role in cancer than anyone had previously considered.
Poore is currently an MD/PhD student at University of California San Diego School of Medicine, where he’s conducting his graduate thesis work in the lab of Rob Knight, PhD, professor and director of the Center for Microbiome Innovation.
Together with an interdisciplinary group of collaborators, Poore and Knight have developed a novel method to identify who has cancer, and often which type, by simply analyzing patterns of microbial DNA — bacterial and viral — present in their blood.
The study, published March 11, 2020 in Nature, may change how cancer is viewed, and diagnosed.
“Almost all previous cancer research efforts have assumed tumors are sterile environments, and ignored the complex interplay human cancer cells may have with the bacteria, viruses and other microbes that live in and on our bodies,” Knight said.
“The number of microbial genes in our bodies vastly outnumbers the number of human genes, so it shouldn’t be surprising that they give us important clues to our health.”
Cancer-associated microbial patterns
The researchers first looked at microbial data available from The Cancer Genome Atlas, a database of the National Cancer Institute containing genomic and other information from thousands of patient tumors. To the team’s knowledge, it was the largest effort ever undertaken to identify microbial DNA in human sequencing data.
From 18,116 tumor samples, representing 10,481 patients with 33 different cancer types, emerged distinct microbial signatures, or patterns, associated with specific cancer types. Some were expected, such as the association between human papillomavirus (HPV) and cervical, head and neck cancers, and the association between Fusobacterium species and gastrointestinal cancers. But the team also identified previously unknown microbial signatures that strongly discriminated between cancer types. For example, the presence of Faecalibacterium species distinguished colon cancer from other cancers.
Armed with the microbiome profiles of thousands of cancer samples, the researchers then trained and tested hundreds of machine learning models to associate certain microbial patterns with the presence of specific cancers. The machine learning models were able to identify a patient’s cancer type using only the microbial data from his or her blood.
The researchers then removed high-grade (stage III and IV) cancers from the dataset and found that many cancer types were still distinguishable at earlier stages when relying solely on blood-derived microbial data. The results held up even when the team performed the most stringent bioinformatics decontamination on the samples, which removed more than 90 percent of the microbial data.
Applying the microbial DNA test
To determine if these microbial patterns could be useful in the real world, Knight, Poore and team analyzed blood-derived plasma samples from 59 consenting patients with prostate cancer, 25 with lung cancer and 16 with melanoma, provided by collaborators at Moores Cancer Center at UC San Diego Health. Employing new tools they developed to minimize contamination, the researchers developed a readout of microbial signatures for each cancer patient sample and compared them to each other and to plasma samples from 69 healthy, HIV-negative volunteers, provided by the HIV Neurobehavioral Research Center at UC San Diego School of Medicine.
The team’s machine learning models were able to distinguish most people with cancer from those without. For example, the models could correctly identify a person with lung cancer with 86 percent sensitivity and a person without lung disease with 100 percent specificity. They could often tell which participants had which of the three cancer types. For example, the models could correctly distinguish between a person with prostate cancer and a person with lung cancer with 81 percent sensitivity.
“The ability, in a single tube of blood, to have a comprehensive profile of the tumor’s DNA (nature) as well as the DNA of the patient’s microbiota (nurture), so to speak, is an important step forward in better understanding host-environment interactions in cancer,” said co-author Sandip Pravin Patel, MD, a medical oncologist and co-leader of experimental therapeutics at Moores Cancer Center at UC San Diego Health.
“With this approach, there is the potential to monitor these changes over time, not only as a diagnostic, but for long-term therapeutic monitoring. This could have major implications for the care of cancer patients, and in the early detection of cancer, if these results continue to hold up in further testing.”
Comparison to current cancer diagnostics
According to Patel, diagnosis of most cancers currently requires surgical biopsy or removal of a sample from the suspected cancer site and analysis of the sample by experts who look for molecular markers associated with certain cancers. This approach can be invasive, time-consuming and costly.
Several companies are now developing “liquid biopsies” — methods to quickly diagnose specific cancers using a simple blood draw and technologies that allow them to detect cancer-specific human gene mutations in circulating DNA shed by tumors. This approach can already be used to monitor progression of tumors for some types of already-diagnosed cancers, but is not yet approved by the U.S. Food and Drug Administration (FDA) for diagnostic use.
“While there has been amazing progress in the area of liquid biopsy and early cancer detection, current liquid biopsies aren’t yet able to reliably distinguish normal genetic variation from true early cancer, and they can’t pick up cancers where human genomic alterations aren’t known or aren’t detectable,” said Patel, who also serves as the deputy director of the San Diego Center for Precision Immunotherapy.
That’s why there’s often a risk that current liquid biopsies will return false-negative results in the setting of low disease burden. “It’s hard to find one very rare human gene mutation in a rare cell shed from a tumor,” Patel said. “They’re easy to overlook and you might be told you don’t have cancer, when you really do.”
According to the researchers, one advantage of cancer detection based on microbial DNA, compared to circulating human tumor DNA, is its diversity among different body sites. Human DNA, in contrast, is essentially the same throughout the body. By not relying on rare human DNA changes, the study suggests that blood-based microbial DNA readouts may be able to accurately detect the presence and type of cancers at earlier stages than current liquid biopsy tests, as well as for cancers that lack genetic mutations detectable by those platforms.
Limitations and cautions
The researchers are quick to point out that there’s still the possibility blood-based microbial DNA readouts could miss signs of cancer and return a false-negative result. But they expect their new approach will become more accurate as they refine their machine learning models with more data.
And while false negatives may be less common with the microbial DNA approach, false positives — hearing you have cancer when you don’t — are still a risk.
Patel said that just because a cancer is detected early, it doesn’t mean it always requires immediate treatment. Some DNA changes are non-cancerous, changes related to aging, harmless or self-resolve. You would never know about them without the test. That’s why more screening and more cancer diagnoses might not always be a good thing, Patel said, and should be determined by expert clinicians.
The team also cautioned that even if a microbial readout indicates cancer, the patient would likely require additional tests to confirm the diagnosis, determine the stage of the tumor and identify its exact location.
Looking ahead
Knight said many challenges still lay ahead as his team further develops these initial observations into an FDA-approved diagnostic test for cancer. Most of all, they need to validate their findings in a much larger and more diverse patient population, an expensive undertaking. They need to define what a “healthy” blood-based microbial readout might look like among many, diverse people. They’d also like to determine whether the microbial signatures they can detect in human blood are coming from live microbes, dead microbes or dead microbes that have burst open, dispersing their contents — an insight that might help them refine and improve their approach.
To advance blood-based microbial DNA readouts through the next steps toward regulatory approval, commercialization and clinical application of a diagnostic test, Knight and Poore have filed patent applications and they founded a spinout company called Micronoma, with co-author Sandrine Miller-Montgomery, PhD, professor of practice in the Jacobs School of Engineering and executive director of the Center for Microbiome Innovation at UC San Diego.
The latest study may prompt important shifts in the field of cancer biology, Poore said.
“For example, it’s common practice for microbiologists to use many contamination controls in their experiments, but these have historically been rarely used in cancer studies,” he said. “We hope this study will encourage future cancer researchers to be ‘microbially conscious.'”
The researchers also suggest cancer diagnostics may only be the beginning for the newly discovered cancer-associated blood microbiome.
“This new understanding of the way microbial populations shift with cancer could open a completely new therapeutic avenue,” Miller-Montgomery said. “We now know the microbes are there, but what are they doing? And could we manipulate or mimic these microbes to treat cancer?”
Additional co-authors include: Qiyun Zhu, Carolina Carpenter, Serena Fraraccio, Stephen Wandro, Tomasz Kosciolek, Stefan Janssen, Se Jin Song, Jad Kanbar, Robert Heaton, Rana McKay, Austin D. Swafford, UC San Diego; Evguenia Kopylova, formerly of UC San Diego, now at Clarity Genomics; and Jessica Metcalf, formerly of UC San Diego, now at Colorado State University, Fort Collins.
Continue Reading
Related Articles
Caloric restriction mimetics enhance anti-tumor efficacy

We’re happy to announce that the Seerave Fellow Dr. Jonathan Pol published part of his work entitled “A synergistic triad of chemotherapy, immune checkpoint inhibitors, and caloric restriction mimetics eradicates tumors in mice” in the journal OncoImmunology.
The lab of Professor Guido Kroemeber has recently shown that chemotherapy with agents inducing immunogenic cell death (ICD), such as anthracyclines (e.g. mitoxantrone) or the platinum salt oxaliplatin, can be advantageously combined with fasting or caloric restriction mimetics (CRMs) to reach a better control of tumor growth (1-3). The antitumor activity of the treatment depended on immune actors, particularly on CD8+ T cells. Among these CRMs, Jonathan Pol was particularly interested in further studying hydroxycitrate (HC) and spermidine (SPD). In this follow up study, Jonathan Pol and colleagues revealed that the myeloid immune compartment is also required for the efficacy of this therapeutic combination (4). Indeed, blocking of the integrin CD11b, which participates in the extravasation of myeloid cells, abrogated the benefit of CRMs to chemotherapy. In-depth characterization of the myeloid and lymphoid immune subpopulations infiltrated into the tumor bed allowed a better understanding of the underlying immune mechanisms. In particular, when combined with chemotherapy, HC and fasting amplified a population of dendritic cells derived from monocytes (moDCs, Ly6ChiLy6G+CD11c+CD11b+) and displaying an activated phenotype (CD80+MHC-IIint/hi). In contrast, complementation with SPD was responsible for an increased infiltration of inflammatory macrophages (F4/80+CD11b+CD11c–CD38+).
These moDCs and macrophages participate in cancer immunosurveillance by promoting the activation of CD8+ T lymphocytes able to eliminate malignant cells. Interestingly, chemotherapy alone doubled the influx of CD8+ T cells into the tumor. In combination with fasting, their infiltration was further increased but was accompanied by a more pronounced exhaustion phenotype, as shown by the expression of the negative immune feedback molecule PD-1 at the surface of CD8+ T cells. In comparison, the introduction of CRMs maintained a CD8+ T cell population of comparable size to chemotherapy alone but less exhausted than with fasting. In contrast, the CRM HC appeared to induce a rise in activated CD8+ T lymphocytes, characterized by the expression of the surface marker ICOS.
In parallel, Jonathan Pol and colleagues found that chemotherapy was inducing the overexpression of PD-L1 (PD-1 ligand) on both cancer cells and leukocytes (typically the myeloid compartment). Thus, the detection of PD-1 and PD-L1, both inhibiting the antitumor activity, on multiple cellular components of the tumor environment prompted us to introduce an immunotherapy blocking the PD-1/PD-L1 axis. The latter treatment relies on the administration of an antibody targeting PD-1 that prevents the interaction with its ligand PD-L1. Three anti-PD-1 immunotherapies are nowadays approved into the clinic: Nivolumab (Opdivo, BMS), Pembrolizumab (Keytruda, MSD), and Cemiplimab (REGN-2810, Sanofi). In our mouse fibrosarcoma model, treatment with anti-PD1 alone or in combination with fasting or CRMs had no significant antitumor effect. In contrast, the combination of anti-PD-1 with chemotherapy provided a benefit comparable to that of CRMs. However, complete regression of the majority of tumors was obtained only by a triple therapy combining (i) ICD-inducing chemotherapy, in this case mitoxantrone or oxaliplatin, (ii) a CRM such as HC or SPD, and substitutable by fasting, and (iii) an antibody blocking PD-1 and PD-L1 interaction. (4).
Overall, these results suggest the possibility of synergistic interactions between distinct classes of anticancer agents. Clinical trials are in preparation to evaluate this therapeutic triad against different malignant indications.
Full article: https://doi.org/10.1080/2162402X.2019.1657375
References:
- F. Pietrocola*, J. Pol*, E. Vacchelli, S. Rao, D. P. Enot, E. E. Baracco, S. Levesque, F. Castoldi, N. Jacquelot, T. Yamazaki, L. Senovilla, G. Marino, F. Aranda, S. Durand, V. Sica, A. Chery, S. Lachkar, V. Sigl, N. Bloy, A. Buque, S. Falzoni, B. Ryffel, L. Apetoh, F. Di Virgilio, F. Madeo, M. C. Maiuri, L. Zitvogel, B. Levine, J. M. Penninger, G. Kroemer, Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell 30, 147-160 (2016).
- F. Pietrocola*, J. Pol* E. Vacchelli, E. E. Baracco, S. Levesque, F. Castoldi, M. C. Maiuri, F. Madeo, G. Kroemer, Autophagy induction for the treatment of cancer. Autophagy 12, 1962-1964 (2016).
- F. Pietrocola*, J. Pol*, G. Kroemer, Fasting improves anticancer immunosurveillance via autophagy induction in malignant cells. Cell Cycle 15, 3327-3328 (2016).
- S. Levesque, J. Le Naour, F. Pietrocola, J. Paillet, M. Kremer, F. Castoldi, E. E. Baracco, Y. Wang, E. Vacchelli, G. Stoll, A. Jolly, P. De La Grange, L. Zitvogel, G. Kroemer, J. G. Pol, A synergistic triad of chemotherapy, immune checkpoint inhibitors, and caloric restriction mimetics eradicates tumors in mice. Oncoimmunology 8, e1657375 (2019).
Continue Reading
Related Articles
Human microbiome-derived bacterial strains with antitumor activity
Vedanta Biosciences, a clinical-stage company developing a new category of therapies for immune-mediated diseases based on rationally defined consortia of human microbiome-derived bacteria, today announced a publication in Nature reporting a newly discovered mechanism underlying antitumor immunity that involves human microbiota-driven induction of interferon-gamma-producing (IFNy+) CD8+ T cell accumulation in the gut and tumors.
Led by Vedanta’s scientific co-founder Kenya Honda, M.D., Ph.D., of Keio University School of Medicine, the research also led to the identification and selection of a defined consortium of human microbiome-derived bacterial strains that harnesses this mechanism of antitumor activity and cooperatively potentiates responses to checkpoint inhibitor therapies and immune challenges in general. Based on this research, Vedanta is advancing VE800, a proprietary clinical candidate designed to enhance immune responses against cancer. Vedanta plans to initiate clinical studies in 2019 to evaluate VE800 in combination with Bristol-Myers Squibb’s checkpoint inhibitor OPDIVO (nivolumab).
“This research demonstrates that specific, human microbiome-derived bacteria assembled rationally into consortia can cooperatively enhance the responses to immune checkpoint inhibitors, which supports our hypothesis that modulating the gut microbiota could be a powerful tool for potentiating immune responses that help fight cancer and infection,” said Bernat Olle, Ph.D., Chief Executive Officer of Vedanta Biosciences. “This work also builds upon Dr. Honda’s previous groundbreaking research on the role of the human microbiome in modulating a range of immune responses and provides a robust scientific foundation for our proprietary lead cancer candidate, VE800.”
The authors of the Nature paper sought to understand the previously poorly characterized relationship between the human microbiota and intestinal IFNy+ CD8 T cells, which are critical to innate and adaptive immune responses. In preclinical models, they were able to establish that the number and frequency of these immune cells in the gut depend on the presence of a gut microbiota and are plastic, with specific members of the microbiota promoting their intestinal accumulation in an inducible and reversible manner. The authors went on to identify specific commensal bacterial strains from healthy human donors that spurred the production of IFNy+ CD8+ T cells.
Through rigorous selection, the authors isolated a defined consortium of commensal bacteria derived from the human microbiome that proved most effective at inducing rapid and persistent accumulation of IFNy+ CD8+ T cells. Mice colonized with the defined bacterial consortium demonstrated enhanced therapeutic efficacy in a range of tumor models when given in conjunction with PD-1 or CTLA4 immune checkpoint inhibitors. The strains identified are primarily rare, low-abundance components of the human microbiome, representing a significant opportunity for amplification as a therapeutic strategy.
The research demonstrates for the first time that human microbiome-derived bacterial consortia that cooperatively enhance the responses of immune checkpoint inhibitors can be identified. The authors addressed the challenge of reducing a complex community of human microbiome bacteria down to a few, rationally defined members that can induce a robust immune potentiation response necessary for an effective cancer immune therapy, and directly linking their activity to pathways that promote antitumor immunity.
The Nature paper also found that human stool samples showed considerable variability in their ability to induce colonic IFNy+ CD8+ T cells. Vedanta’s development process is designed to bypass this variability by using pure, clonal cell banks of well-characterized bacterial strains isolated from healthy humans to produce defined consortia of uniform composition. This eliminates the need to rely on direct sourcing of fecal donor material of inconsistent composition. Vedanta sources bacteria from a vast, extensively characterized collection of 80,000 bacterial isolates obtained from human donors from four continents, which is believed to be the largest collection of human-gut associated bacteria. It then designs high-throughput assays to screen product candidates against a given disease target.
VE800 is Vedanta Biosciences’ proprietary oral immuno-oncology product candidate. It consists of a rationally defined bacterial consortium that activates cytotoxic CD8+ T cells, a type of white blood cell that is the predominant effector in cancer immunotherapy. In preclinical studies, VE800 has been shown to enhance the ability of these T cells to infiltrate tumors, thereby promoting suppression of tumor growth and enhancing survival. Data also suggest that VE800 may enhance the effects of checkpoint inhibitors. Vedanta is evaluating VE800 alone and in combination with checkpoint inhibitors as a potential treatment for patients with advanced or metastatic cancers. In December 2018, Vedanta entered into a clinical trial collaboration to evaluate Bristol-Myers Squibb’s programmed death-1 (PD-1) immune checkpoint inhibitor OPDIVO (nivolumab) in combination with Vedanta’s VE800, in patients with advanced or metastatic cancers. Clinical trials are expected to begin in 2019.
Story Source: medicalexpress.com
More information: https://doi.org/10.1038/s41586-019-0878-z
Continue Reading
Related Articles
Gut microbiome regulates the intestinal immune system

A new study in mice unveils the role of vitamin A in immune system regulation, a finding that could assist in developing treatments for autoimmune and inflammatory diseases as well as vitamin A deficiency.
PROVIDENCE, R.I. [Brown University] — Scientists have long known that bacteria in the intestines, also known as the microbiome, perform a variety of useful functions for their hosts, such as breaking down dietary fiber in the digestive process and making vitamins K and B7.
Yet a new study unveils another useful role the microbiome plays. A team of researchers from Brown University found that in mice, the gut microbiome regulates the host’s immune system — so that rather than the host’s defense system attacking these helpful bacteria, the bacteria can coexist peacefully with the immune system.
What’s the trick to the microbiome’s work with the immune system? Vitamin A — the bacteria moderate active vitamin A levels in the intestine, protecting the microbiome from an overactive immune response.
That insight may prove important for understanding and treating autoimmune and inflammatory diseases, said Shipra Vaishnava, an assistant professor of molecular microbiology and immunology at Brown.
“A lot of these diseases are attributed to increased immune response or immune activation, but we’ve found a new way that bacteria in our gut can dampen the immune response,” Vaishnava said. “This research could be critical in determining therapies in the case of autoimmune diseases such as Crohn’s disease or other inflammatory bowel diseases, as well as vitamin A deficiency.”
The study was published on Tuesday, Dec. 18, in the journal Immunity.
Microbiomes of mice and men
The gut microbiome is an ecosystem made of 100 trillion bacteria that have evolved to live in the special conditions of the intestines, Vaishnava said. The vast majority of these bacteria are helpful rather than harmful. A healthy microbiome, just like a healthy forest, has many species coexisting together and can fend off hostile intruders — such as disease-causing bacteria or invasive species.
In both humans and mice, the phyla Firmicutes and Bacteroidetes comprise the majority of the gut microbial community. To play their part in regulating their hosts’ immune systems, the bacteria in the microbiome fine-tune the levels of a protein responsible for the conversion of vitamin A to its active form in their hosts’ gastrointestinal tract, the researchers found.
Vaishnava’s team found that Firmicutes bacteria, particularly members of the class Clostridia, reduce the expression of a protein within the cells that line the intestines. The protein, retinol dehydrogenase 7 (Rdh7) converts dietary vitamin A to its active form, retinoic acid, Vaishnava said. The Clostridia bacteria, common to both mice and men, also promote increased vitamin A storage in the liver, the team found.
Vaishnava expects the findings are generalizable to the interactions between the human microbiome and their hosts as well.
Mice genetically engineered to not have Rdh7 in their intestinal cells have less retinoic acid in the intestinal tissue, as the researchers expected. Specifically, the guts of the engineered mice had fewer immune cells that make IL-22, an important cellular signal that coordinates the antimicrobial response against gut bacteria. Other components of the immune system such as cells with immunoglobulin A and two types of T-cells were the same as in standard mice, suggesting Rdh7 is only essential for the regulating antimicrobial response, Vaishnava said.
The researchers do not know exactly how Rdh7 is suppressed, but Clostridia bacteria are known to produce short chain fatty acids that change host gene expression. As a next step in their research, the team will study how bacteria regulate Rdh7 expression, including examining various short chain fatty acids, Vaishnava said.
In addition, the team will conduct research to understand why Rdh7 suppression is critical. They are working to genetically engineer mice to always express Rdh7 in their intestinal cells. Vaishnava wants to see how this affects the mouse microbiome and if it leads to any inflammation or autoimmune disease-like conditions for the mice. They will also explore the impacts of increased vitamin A storage in the liver due to bacteria Rdh7 regulation, Vaishnava said.
Helping human health
The researchers say that understanding how bacteria regulate the immune system’s responses could be important in unlocking the keys to disorders like Crohn’s disease.
Data from clinical studies has shown that inflammation in the bowel is a result of disrupted interactions between a host and their gut microbiome, Vaishnava said.
“The role of vitamin A in inflammation is context-dependent and is very hard to tease apart,” Vaishnava said. “A change in vitamin A status and vitamin A metabolic genes coincides with inflammatory bowel diseases, but we don’t know if this promotes inflammation or not. We hope that adding our finding — that bacteria can regulate how vitamin A is being metabolized in the intestine or stored — could help clarify why the field is seeing what it is seeing.”
These findings could also provide clues about the importance of the microbiome in addressing vitamin A deficiency, a problem that is particularly prevalent in Africa and Southeast Asia.
Vitamin A deficiency affects approximately one third of children under the age of five, according to the World Health Organization (WHO). Vitamin A deficiency weakens the immune system and increases the risk of infectious diseases. The WHO has been providing at-risk children with vitamin A supplements for the past 25 years, but it hasn’t been as successful as hoped for, according to Vaishnava. This study shows bacteria are a big part of vitamin A absorption and storage and perhaps children need to have the right combination of bacteria in the gut in order for the vitamin A supplements to be most effective, she added.
“Both our diet and the bacteria in our gut are critically linked in regulating how our immune cells behave,” Vaishnava said. “Finding what those links are at a molecular level is important to figuring out how we could use either diet or bacteria, or both of them together, to have a therapeutic effect in inflammatory or infectious diseases.”
In addition to Vaishnava, other authors from Brown include Mayara Grizotte-Lake, Kellyanne Duncan, Namrata Iyer and Irina Smolenski. Authors also include Nina Isoherranen, Guo Zhong and Jay Kirkwood from the University of Washington, Seattle.
The National Institutes of Health (grants R01-DK113265 and P20-GM10903) and the Crohn’s and Colitis Foundation of America supported the research.
Story Source: https://news.brown.edu/articles/2018/12/microbiome
More information: https://doi.org/10.1016/j.immuni.2018.11.018
Continue Reading