Scientists from Switzerland and the US have shown that lymphatic vessels can enable both metastasis and T-cell invasion, opening new paths for cancer immunotherapy.
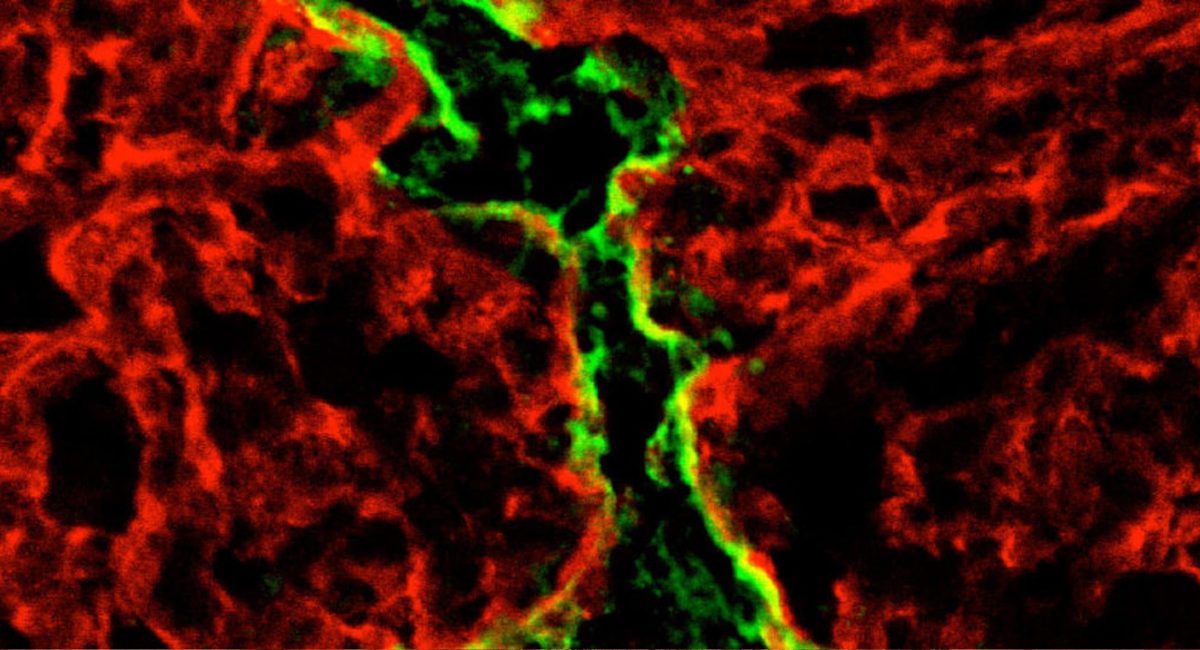
Many cancers, such as melanoma, are known to metastasize and spread by expanding nearby lymphatic vessels. This process, lymphangiogenesis, also helps the tumor evade the patient’s own immune system, and it would be expected that inhibiting lymphangiogenesis, could enhance the efficacy of cancer immunotherapies, which are only effective in a minority of patients. But in a surprising discovery, scientists from EPFL and the US found the opposite: lymphangiogenesis actually enhances the effectiveness of immunotherapy against melanoma. Published in Science Translational Medicine, the study has significant implications for new types of cancer therapies.
Cancer immunotherapy is one of the most promising treatments against tumors. The process involves overcoming the tumor’s suppression of immune attacks, thus allowing the patient’s own immune system to destroy it. But despite the highly encouraging results from clinics, only a subset of patients is able to respond to immunotherapy. Until now, the reasons have been unclear.
One of the problems is that many tumors evolve clever defenses against the patient’s immune system to evade or survive such attacks. For example, melanomas and other tumors induce lymphangiogenesis with a protein called Vascular Endothelial Growth Factor C (VEGF-C). The presence of VEGF-C and subsequent lymphangiogenesis are generally signs of metastasis and create a poor prognosis for the patient. In addition, recent studies have also suggested that VEGF-C may also help the melanoma tumor suppress the patient’s immune system.
Consequently, a team of scientists led by the lab Melody Swartz at EPFL (now at the University of Chicago) with co-first authors Manuel Fankhauser and Maria Broggi hypothesized that VEGF-C induced lymphangiogenesis and immune suppression would hinder the effectiveness of immunotherapy.
Surprisingly, the scientists found that VEGF-C and lymphangiogenesis can strongly boost the effects of immunotherapy in melanoma. The discovery was unexpected, as the scientists were trying to enhance immunotherapy by blocking VEGF-C in mouse melanoma models. Instead, they observed the opposite: the impact of immunotherapy on mice with melanoma actually got worse when lymphangiogenesis was blocked.
Following up with further studies, the researchers found an explanation for their observations: the new lymphatic vessels created by the melanoma tumor secrete the chemokine CCL21, which actively attracts naïve (undifferentiated) T cells into the tumor’s immunosuppressed microenvironment. Once in the tumor, these naïve T cells are locally activated following immunotherapy-induced tumor cell death, which triggers a positive feedback loop of long-lasting anti-tumor immunity.
The team tested their hypothesis on different mouse models of melanoma using multiple immunotherapy approaches, including vaccination and adoptive T cell transfer. All of these eradicated or slowed down the growth of primary melanoma tumors, and in some settings even conferred the mice with long-term protection against metastasis.
Beyond the pre-clinical models though, the scientists tested their hypothesis in human patients with melanoma. “The difference was really striking,” says Melody Swartz. “Almost all of the patients with higher than average VEGF-C levels in their blood responded to immunotherapy. This not only resulted in eradication of the primary tumors, it also encouraged T cell infiltration into metastatic tumors and resulted in long-term protection.” The researchers therefore propose that VEGF-C can be a predictive biomarker that indicates how well a patient is responding to immunotherapy.
“We now appreciate the numerous mechanisms of immunosuppression that a T cell-inflamed tumor develops to survive, including lymphangiogenesis,” the authors write. “But when the scales are tipped toward activating factors dominating over suppressive ones, as is the case with immunotherapy, these T cells become robust participants in antitumor immunity.”
The findings reveal an unappreciated role of tumor-associated lymphangiogenesis in shaping the tumor’s immune microenvironment, and pave the way for therapeutic strategies that exploit it. The study is of critical importance to cancer immunotherapy, which is growing into a formidable weapon against tumors of different types. For example, the FDA recently approved CAR-T cell therapy for leukemia and several checkpoint inhibitors are already approved for the treatment of solid tumors such as melanoma. All of these are breakthrough medicines that are now curing patients who would previously have had low chances for survival.
Story source: EPFL News
Article: Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Science Translational Medicine (2017). Fankhauser, M., Broggi, M. A. S., Potin, L., Bordry, N., Jeanbart, L., Lund, A. W., et al.
http://doi.org/10.1126/scitranslmed.aal4712