Science Philanthropy / Rutgers University
Restoration of Antibiotics Effects on the Infant Microbiota by Autologous Fecal Transplant
Background
Antibiotics have saved many lives in the last 70 years. However, while antibiotics are successful in eradicating many pathogenic bacteria, research has demonstrated a deleterious effect on the microbiome, which in infants translates in retarded microbiome maturation, physiological effects that include increased risks of later immune and metabolic disorders. Strong epidemiological evidence suggests associations between early stressors of the microbiota and a number of common diseases, such as obesity , asthma , allergies, celiac disease, and T1D. Furthermore, excess antibiotic exposure is associated with the development of neurological and psychiatric disorders.
Given not only the tendency of early perturbations to increase disease risks, but also the substantial increase in global use of antibiotics, it is important to evaluate effects of significant perturbations to microbiome at early ages, such as through antibiotic use. There are enormous variations in antibiotics use, with some countries recording as high as 12 courses a year in children younger than two. Currently, no strategies exist to restore the microbiome of infants that need to be treated for bacterial infections, other than reliance on spontaneous recovery, which is known to be slow and incomplete.
A new therapeutic practice has been approved to treat C.difficile intestinal infections (which are refractory to antibiotics), and that is fecal microbiota transplantation (FMT). FMT has been extensively shown to be restorative in adults, consistent with the fundamental ecological principle that mature communities are more resilient than perturbed or immature communities. Other uses of microbiota restoration are being researched, including restoring the microbiome of infants born via C-sections with maternal FMT or maternal vaginal transplant, which has been shown to be effective as well.
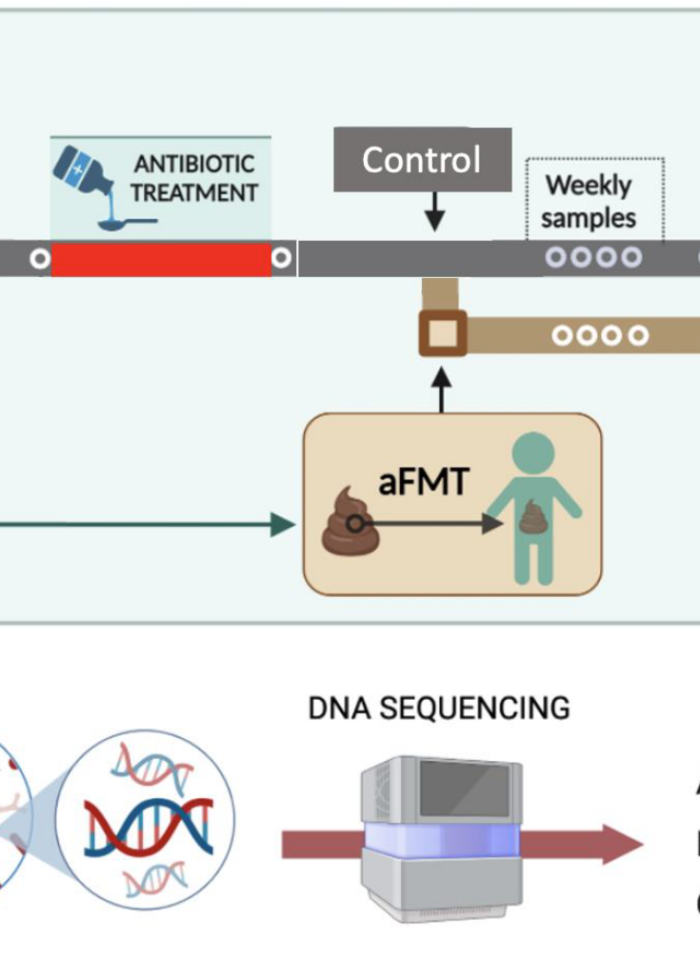
Autologous FMT (aFMT) lacks the concerns of horizontal transmission of infectious microbes associated with heterologous transplantation. In mice, fecal autologous transplant following antibiotics showed full restorative effect bouncing the microbiome to the pre-antibiotic structure and, contrary to popular belief, ingestion of probiotics after antibiotics has been demonstrated to slow down post-antibiotic recovery. Many babies are being given prebiotics, particularly after antibiotic courses. Building on these and other findings, testing the restorative capacity of aFMT on disrupted microbiomes in human babies is of great clinical importance, particularly because they are on a crucial developmental stage.
We hypothesize that reintroducing the pre-antibiotic microbiome from a healthy child after an antibiotic course leads to a faster and more effective recolonization than spontaneous restoration. We expect a fast bounce back of the microbiota structure to the pre-antibiotic conformation and normalization of microbiome maturation, while non-restored children will maintain an aberrant microbiome for a longer period of their development. If this hypothesis is confirmed, future studies to determine the effect on reducing the antibiotics-associated disease risks will be important.
Goals
To determine the speed and extent of post-antibiotic gut microbiome restoration in children receiving or not an oral autologous fecal transplant.
Dominguez-Bello Lab

Gloria Dominguez-Bello
Principal Investigator
Dr. Dominguez-Bello focuses on the microbiome development from birth, functions for the host, impact by practices that reduce microbial transmission or disrupt the microbiota, and strategies for restoration. She also studies how Westernization changes environmental microbes and human exposures, integrating the fields of anthropology and architecture/urban studies into microbial ecology.

Anna Dulencin
Collaborator
Anna Dulencin is an Associate Research Professor and Director of the Eagleton Science and Politics Program at Rutgers University. Following a BA in Genetics and PhD in Neuroscience also from Rutgers, she was a biomedical research consultant at the New Jersey Autism Center of Excellence. As a Visiting Fellow in the Department of Biochemistry and Microbiology, she studies the infant microbiome and workforce strategies to support maternal-infant bonding with the aim of lowering early-childhood microbiome disturbances due to antibiotics and maternal separation.